特罗安-戈蒂耶教授:重新定义超快瞬态吸收的可及性
“This technology represents one of the biggest breakthroughs of this century that renders ultrafast transient absorption spectroscopy available to both beginners and experienced users,” says Ludovic Troian-Gautier, professor at Université catholique de Louvain (UCLouvain).
Prof. Troian-Gautier graduated from the Université libre de Bruxelles, where he focused on the development of novel transition metal complexes for optoelectronic applications. During his post-doctoral research, he also worked on surface modification, the development of novel molecular photocatalysts, and their application in novel photoelectrodes capable of generating so-called “solar fuels.” He currently continues his research on energy-related challenges at UCLouvain – one of the most highly ranked universities in Belgium, recently placed among the top 200 worldwide – and was among the first to explore the capabilities of Light Conversion’s HARPIA-LIGHT tabletop transient absorption spectroscopy system.
At UCLouvain, Prof. Troian-Gautier’s research group focuses on multiple facets of photochemistry, with three main research directions:
The investigation of bimolecular excited-state electron transfer and the mechanisms governing the separation of radical species following electron transfer
For two molecules to react, they must first diffuse and form an encounter complex, which is a prerequisite for excited-state electron transfer. This mechanism is commonly referred to as dynamic quenching. In the case of short-lived excited states, it is possible to pre-associate the molecules in the ground state, thereby eliminating the need for diffusion. This leads to static quenching, where the quenching process occurs on the ultrafast timescale.
Following excited-state electron transfer, two radicals are formed and confined within a solvent cage. The factors governing whether these radicals escape the cage and evolve into independently solvated radicals – useful in many aspects of chemistry and biology – remain poorly defined. This challenge represents the major research focus of Prof. Troian-Gautier’s group.
They have demonstrated that the efficiency of cage escape controls the overall yield of a light-mediated chemical reaction. Therefore, gaining detailed insight into the cage escape process – particularly through the use of ultrafast transient absorption spectroscopy for pre-associated species – will undoubtedly contribute to fundamental knowledge. These insights will have immediate implications in many fields that rely on photo-induced electron transfer, such as photoredox catalysis, photochemotherapy, and solar fuel formation.
The development of photoelectrodes using photosensitizers based on earth-abundant elements
These photoelectrodes are developed with the aim of producing sustainable solar fuels, such as hydrogen from protons, CO₂ reduction products, or halogens generated via halide oxidation, among others. The approach used herein is well established in the literature and is analogous to the static quenching mechanism, with the key distinction that the photosensitizer is now anchored to a metal oxide, which acts as the quencher.
Upon light excitation, the excited photosensitizer can inject an electron into the conduction band of the metal oxide, thereby generating an electrical current and forming an oxidized photosensitizer to drive solar fuel formation.
One of the main challenges associated with photosensitizers based on earth-abundant elements is their typically short-lived excited states, which cannot be monitored via nanosecond transient absorption spectroscopy. Furthermore, after electron injection, recombination – back electron transfer from the injected electron to the oxidized photosensitizer – is both rapid and efficient, significantly reducing the overall efficiency of the system.
By employing ultrafast transient absorption spectroscopy, the group can monitor these processes in real time and develop strategies to mitigate such detrimental recombination pathways.
The development of novel photosensitizers based on earth-abundant elements
Photochemistry has long been dominated by photosensitizers based on transition metals such as Ru(II), Ir(III), and Os(II), offering exceptional properties that can be readily tuned. However, their scarcity has become a growing concern – particularly among funding agencies and, by extension, review panels.
In contrast, industry has been comparatively less affected by these concerns, as the cost–benefit ratio remains the primary metric of evaluation. For example, catalytic converters contain platinum, palladium, and rhodium – some of the rarest elements – due to their efficiency in converting toxic gases into CO₂. In these cases, the performance benefit clearly outweighs the cost.
Academia, however, is well-positioned to lead the transition toward the use of earth-abundant elements. Pioneers in the field have already laid a strong foundation, providing design principles that guide further progress. In line with this direction, Prof. Troian-Gautier’s group is focused on developing novel photosensitizers based on earth-abundant metals, with a primary emphasis on iron.
A notable achievement in this effort is the work led by Dr. Felix Glaser, who recently developed an iron–anthracene dyad featuring a non-luminescent excited-state lifetime of 11.5 microseconds. This system enables singlet oxygen 1O₂ formation and enhances cage escape processes. Despite the unusually long excited-state lifetime, ultrafast transient absorption spectroscopy was crucial in resolving the excited-state landscape that led to the incredible increase in lifetime.
Recently, the group has also begun pursuing space-based photochemistry applications, in collaboration with Jaroslav Hruby (Hasselt University) and Anna Ermakova (Hasselt University and Royal Institute for Space Aeronomy), with experiments scheduled to be conducted aboard the International Space Station in the summer of 2026. This multidisciplinary research project aims to develop a diamond-based quantum sensor that, in combination with laboratory-developed photosensitizers and model quenchers, will enable a better understanding of the effects of weightlessness on excited-state electron transfer and pH dynamics. The findings are expected to contribute valuable insights into astrochemical processes occurring in space.
Bridging the Timescale Gap: Making Ultrafast Spectroscopy Accessible
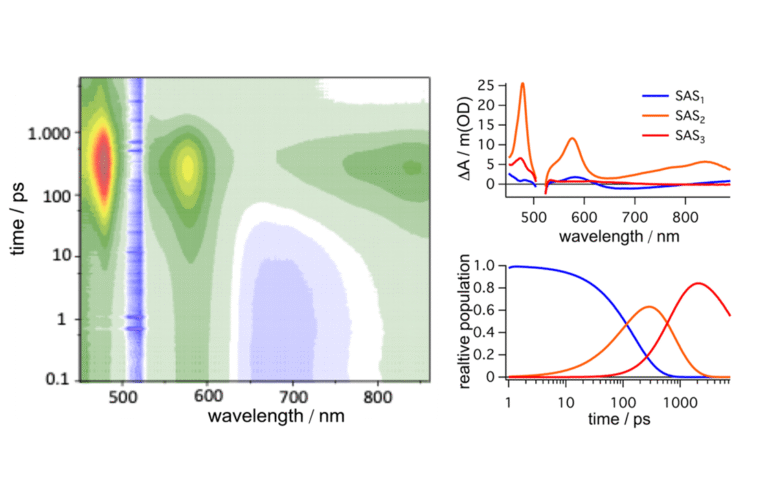
For the different research directions, the group employs a range of spectroscopic techniques, including nanosecond UV-visible transient absorption spectroscopy, nanosecond time-resolved IR spectroscopy, time-correlated single photon counting, and (spectro)electrochemical methods. These experimental techniques provide insights into excited-state dynamics and relaxation pathways, including interfacial or bimolecular electron transfer photochemistry at the early nanosecond timescale. While this timescale is valuable for understanding certain factors that control efficiency, many processes occur at much shorter – femtosecond-to-picosecond – timescales that Prof. Troian-Gautier’s group is currently unable to monitor.
The group performs ultrafast transient absorption spectroscopy measurements in collaboration with Alejandro Cadranel and his dedicated team – a necessity, as this technique is highly sensitive and requires specialized optical tables, clean-room protocols, and precise experimental arrangements. In most cases, such ultrafast setups are operated by a dedicated specialist who ensures optimal measurement conditions and can troubleshoot potential issues as they arise.
“These are obvious limitations that are faced by several laboratories worldwide, which have given ultrafast transient absorption the status of a ‘specialty’ technique and are often more associated with frustration and troubleshooting than actually generating meaningful data. But this ends today! The HARPIA-LIGHT tabletop transient absorption spectroscopy system from Light Conversion is a true revolution in the field,” shares Prof. Troian-Gautier.
From Installation to Insight: Transforming Iron Photochemistry with HARPIA-LIGHT
Upon receiving the HARPIA-LIGHT system, the group was able to measure excited-state dynamics at ultrafast timescales, enabling the study of excited-state electron transfer in pre-associated photosensitizer–quencher pairs, as well as excited-state reactivity on metal oxide thin films using photosensitizers based on earth-abundant elements. HARPIA-LIGHT features two excitation wavelengths – 343 nm and 515 nm – allowing excitation of most photosensitizers used by the group. Additionally, it enables measurements of excited-state dynamics ranging from 290 femtoseconds to 7.5 nanoseconds. The detection wavelength range of 460 to 910 nm supports monitoring of the majority of transient processes occurring within these early timescales.
“Having had first-hand experience with the HARPIA-LIGHT and having unboxed it myself with one of Light Conversion’s exceptional employees, I can certify that the installation is extremely fast and user-friendly. Within two hours, the equipment was installed, calibrated, and we could already measure our sample. The equipment is so user-friendly that in the short time we had it available, 7 users were capable of obtaining meaningful data and modeling the experimental results using the proprietary program installed with the equipment. This would not have been possible using most conventional ultrafast transient absorption setups,” says Prof. Troian-Gautier.
Ultrafast electron or energy transfer processes govern the excited-state landscape, which the group was able to study efficiently using the HARPIA-LIGHT system. The team developed a dedicated research project focused on iron photosensitizers – an ideal match for the system, as these compounds absorb around 515 nm and typically exhibit short excited-state lifetimes. They observed excited-state electron transfer in iron photosensitizers pre-associated with quenchers, eliminating the need for diffusive processes that often dominate solution-based chemistry. The group also investigated iron dyads, where the iron photosensitizer is covalently linked to an energy acceptor. Finally, they studied iron photosensitizers anchored on metal oxide thin films, which enabled the observation of rare excited-state reactivity and prompted a redirection in their research approach using these systems.
“This would not have been possible without the use of the HARPIA-LIGHT. As a result of the data obtained in the short timescale of our use of the setup, we were able to obtain key information on our iron photosensitizers. This has provided us with a deeper understanding of how electrons are transferred and has already changed our research avenue to develop more efficient systems,” claims Prof. Troian-Gautier.
“I truly believe that the HARPIA-LIGHT tabletop transient absorption spectroscopy system has all the key performance to become a best-seller, as it caters not only to specialists but also to less familiar users and beginners who wish to enter the field. The time resolution, ease of installation, and ease of use make this instrument a tabletop alternative to most complicated setups.”